An end to all this prostate trouble?
The prostate gland causes entirely too many problems. In the US,
prostate cancer kills about one man of every forty. “Benign
prostate hyperplasia” (BPH) is even more common, affecting most
men over age 60. It pinches the urinary tract, making it hard to
urinate, and is constantly in danger of transforming from
“benign” to “malignant”. Even the difficulty urinating is enough
of a problem that men often get surgery for it, usually TURP, a
sort of roto-rooter job (except cutting through the pipe instead
of just cleaning it out).
In women, breast cancer has a similar death toll, but the breasts
have an excuse: they’re much bigger; there are many more cells to
go bad. They’re also much more metabolically active, capable of
producing enough milk to feed a baby; the prostate’s output is
tiny in comparison.
One idea I’ve seen is that the prostate serves as the body’s
“gatekeeper” against sexually transmitted diseases, and in the
process often gets chronically infected itself; the resulting
inflammation may cause hyperplasia, first benign then malignant.
Infectious causation is too often neglected these days, and
sexually transmitted diseases are common, so this is not
unreasonable. But it doesn’t seem like a great explanation. The
prostate doesn’t filter the urinary tract; it just secretes into
it; there’s no real “gate” there to be “kept”. The prostate’s
position is like that of the salivary glands, which are not known
for being great houses of cancer. And the epidemiology backs
this up: correlations with STDs are there, but not huge. The
odds ratios are between one and two; in comparison, the odds
ratio for human papillomavirus and oropharyngeal cancer is
greater than ten. An odds ratio of ten should make the ears perk
up; an odds ratio of 1.5 can be a minor effect or can be just a
spurious correlation. And with the prostate, there’s not just a
minor effect which needs explaining: there has to be some
major-league cause.
A decade ago, Scott Alexander, in his blog “Slate Star Codex”,
wrote:
“About five years ago, two Israeli doctors named Gat and
Goren posit the theory that benign prostatic hyperplasia,
a prostate disease that affects millions of older men, is
caused by incompetence of the spermatic veins. They claim
they can treat it surgically, and show off rows of
smiling patients with glowing testimonials. Once again,
the guys are good doctors, nothing about their theory
contradicts basic laws of biology, and no one else has
any better ideas.I shamefacedly admit I want this one to be true. There’s
so much “well, everything is a complicated combination of
genes, biomolecules, biopsychosocial stressors and immune
modulators that we may never really understand” going on
in medicine today that it would be super gratifying if
this one mysterious disease turned out to just be
plumbing going in the wrong direction. And although the
prostate is about as far from my area of expertise as it
is possible to be, I have to say that from a
physiological standpoint their theory seems to have that
rare and much-sought scientific elegance, where
everything comes together in a pretty package.As far as I can tell, the medical community has totally
ignored this one. Gat and Goren have published their
hypothesis and their apparent excellent results in
peer-reviewed medical journals. It has garnered praise
from prestigious figures in the field (bonus points for
calling it “seminal”, especially if the pun was
intentional). As far as I know, no one has attacked it or
even formally expressed doubt. Yet as far as I know, it
has gone nowhere.Does everyone mutually assume that if something this
revolutionary were true, someone would have noticed beyond a
single article in a urology journal? Do they just decide it needs
further research, and hope that this research will be conducted
by someone else? Or do they think that it would end up like
Zamboni’s MS cure, with hundreds of thousands of dollars wasted,
dozens of unnecessary surgeries performed, and nothing to show
but yet another fringe medical idea that sounded good at the
time?
I read that some time after it was written, and found that
there’s since been a small confirmatory study from Germany.
So it hasn’t been entirely neglected. Gat and Goren also have
other papers in the area. (The latter author is sometimes listed
as “Gornish”, that being the non-Hebraicized verson of his name.)
At any rate, Scott’s was more of a social view of the question
than a technical one. Still, it was intriguing enough to get me
to read the papers he linked to, and then to read the authors’
other papers on the subject. I found that the above language
does not convey the full scope of the theory. This is not just
about BPH; it’s also about prostate cancer, and also about
varicocele, the top cause of male infertility. And it promises
to eliminate all these problems, if caught early enough, which is
not hard: screening for it is simple and cheap.
The theory here is largely mechanical; and it’s not just
psychiatrists like Scott who are weak at mechanical explanations;
it’s doctors in general as well as medical researchers and
biologists. There is even a famous paper “Can a Biologist Fix a
Radio?”, wherein the biologist author laments the unsuitability
of biological reasoning, at least of the usual sort, for the task
of fixing an old radio. (End result: “the radio remains
broken.”) Reading medical review papers, I have often gotten
quite disgusted at the way they list result after result in the
fashion “X has an effect on Y”, without saying what the size of
the effect is or even what dose of X is required. Especially
when you want to chain two mechanisms together (X having an
effect on Y and then Y having an effect on Z), you’ve got to know
the numbers. The convention is that p=0.06 means “it doesn’t
have an effect” and p=0.04 means “it has an effect”, but any real
thought requires more than just that binary view of things.
I’m thoroughly comfortable with mechanical theory and practice,
so here’s an attempt at a full rendering of the Gat/Goren theory,
in language as simple as the subject permits, followed by my own
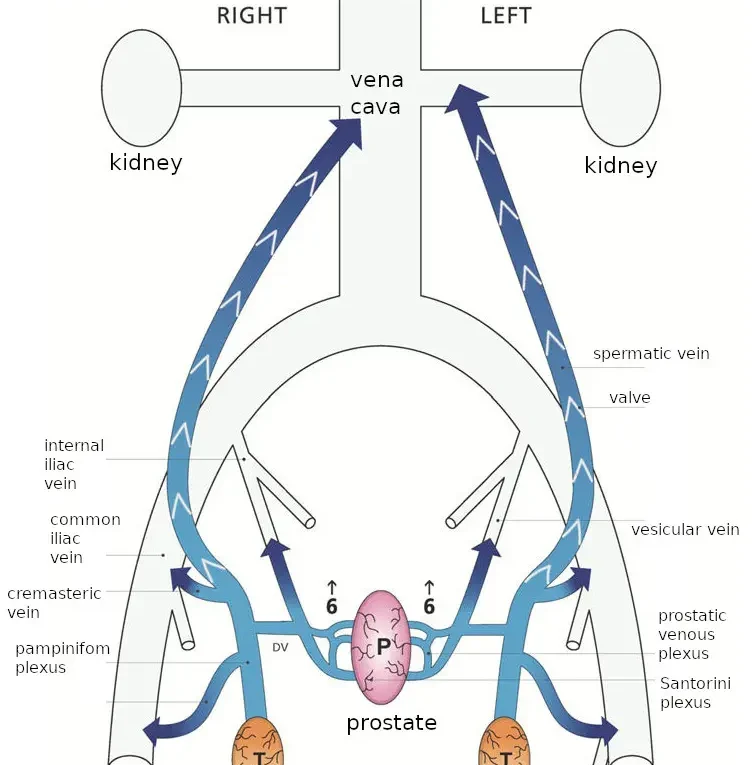
comments. The basic idea is this: in healthy men, blood flows
out from the testicles into the spermatic veins (of which there
is one for each testicle). Each spermatic vein goes up
vertically inside the body until it’s near the kidney on that
side. (The kidneys are about halfway up the back.) The left
spermatic vein then feeds into the left kidney vein, while the
right spermatic vein feeds directly into the vena cava (the vein
“as big as a cave”; it’s the largest vein in the body, leading
directly back to the heart; the kidney veins also feed into it).
Each spermatic vein has about seven one-way valves to prevent
blood from flowing the wrong way through the vein, which it would
otherwise do due to the force of gravity. The following diagram
from their latest paper shows the situation in healthy men.
(That paper is under a Creative Commons license, which this
re-use is in accordance with; I’ve slightly altered their diagram
to un-abbreviate the labels. The subsequent images are also
re-used under the same license.)
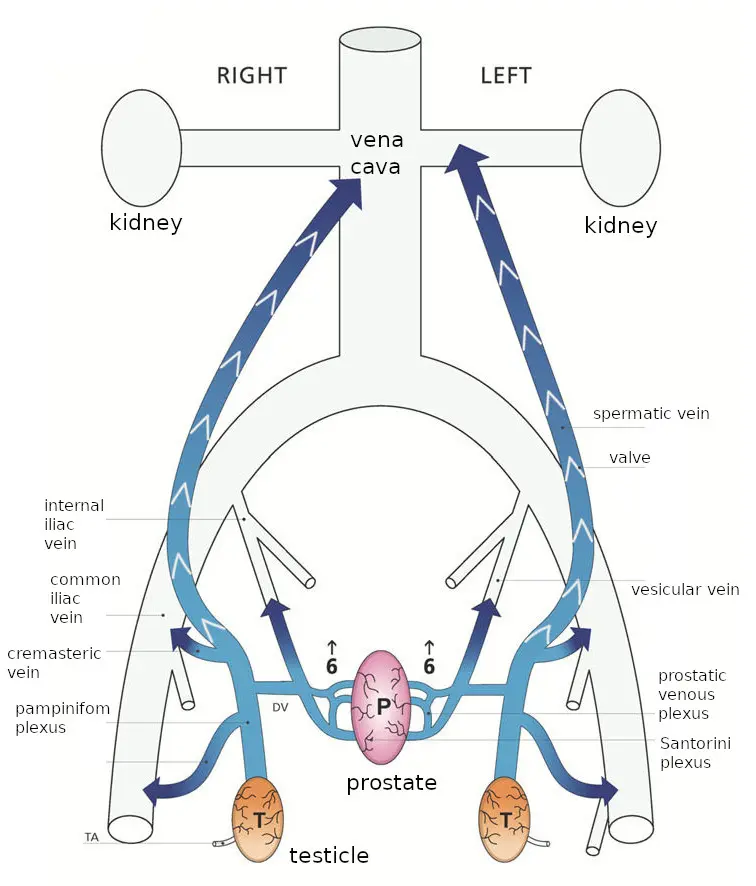
With age and wear and tear, the one-way valves cease to function,
and blood does flow the wrong way: down the vein towards the
testicles. It bathes them in poorly-oxygenated blood, which is
bad for them, eventually killing the germ cells which produce
sperm and causing infertility.
The medical literature often blames that infertility on the
warmth which is produced by that reverse blood flow, but Gat and
Goren blame the low oxygen: warmth does interfere with sperm
production, but it’s low oxygen that actually kills
sperm-producing cells, as occurs in men with this disorder. (The
warmth is still quite useful for diagnosing the disorder since
thermal imaging shows it plainly.)
The blood from the testicles, still at higher than normal
pressure, then continues on out the network of veins in that
area, and spills into the prostate, going backwards through veins
that normally drain the prostate; to use their diagram from the
same paper:
Since this blood has just left the testicles, it is heavily
loaded with testosterone, which promotes prostate growth. Making
things even worse, that blood has a much higher than normal
percentage of free testosterone. Normally the vast majority of
testosterone in the blood is bound to sex hormone binding
globulin (SHBG); only a tiny proportion is free, but that
proportion has the actual hormone effect. In the testicular
flow, there isn’t enough SHBG to bind to all the testosterone;
that happens only on the way back to the heart when it is mixed
with the blood returning from the rest of the body. So the total
effect is to hit prostate cells with about a hundred times the
normal dose of free testosterone. This makes them grow; their
growth is called benign prostatic hyperplasia or prostate cancer.
There’s also another effect of the high pressure: swelling the
prostate (pressurizing it and expanding it). It also swells the
veins in the scrotum, the “pampiniform plexus” above the
testicles; when that swelling is prominent enough, it is given
the name “varicocele”.
(I’d originally imagined that their reason for the high free
testosterone was that the testosterone molecules took time to
find the SHBG molecules and bind to them, but that process seems
to be too fast: this study found a half-life of 12 seconds
for dissociation, and at equilibrium the rate of association must
equal the rate of dissociation. So overwhelming the local supply
of SHBG seems like a better explanation, though being out of
equilibrium might add to it a bit.)
This theory can explain why giving men testosterone doesn’t seem
to increase the risk of prostate cancer, despite testosterone
promoting prostate cancer. Giving men testosterone shuts down
their own production of testosterone, making backflow from the
testicles to the prostate harmless. Thus even with more
testosterone in their blood, they can have less in the prostate
gland.
It can also explain why low testosterone is correlated with
prostate cancer: the backflow damages the testicles via hypoxia,
lowering testosterone, while simultaneously funneling the
testosterone that still is released directly to the prostate.
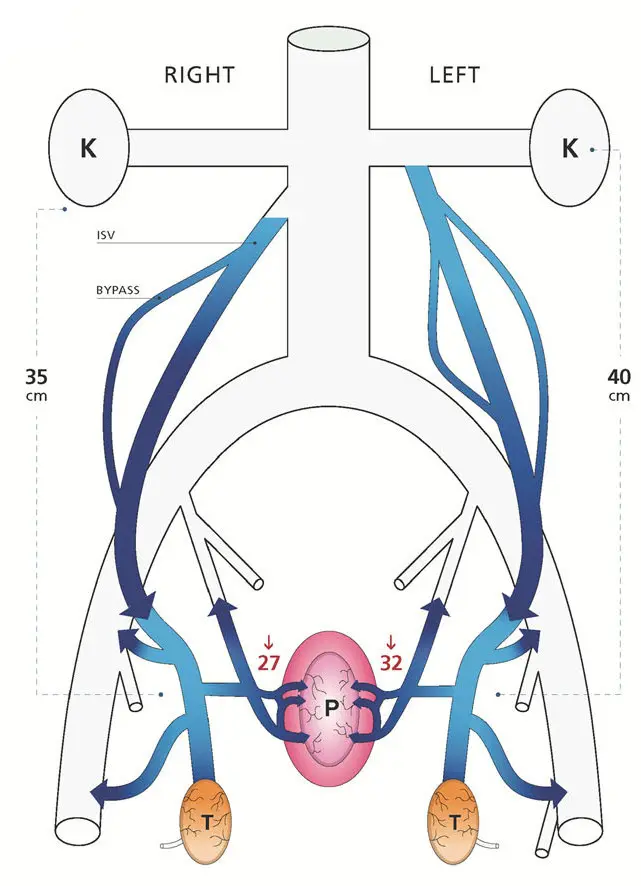
The way Gat and Goren fix this is simply by destroying the
spermatic veins. Under fluoroscopy (that is, viewing an X-ray
image continuously during the procedure), they snake a catheter
in through the veins until it gets to the top of the spermatic
vein. They then inject a pulse of X-ray contrast agent to make
the flow in that vein visible under the fluoroscope, proving that
the flow really is going the wrong way. Having done so, they
then have the patient close off the bottom of the vein with
finger pressure while they inject a sclerosing agent into the
vein; they start by moving the catheter to the bottom and
sclerosing that portion, then repeatedly withdraw the catheter a
bit (moving up) and inject more. After a few minutes the vein is
thoroughly and permanently clogged. This is their diagram of the
results:
To say a bit more about the background of this theory, venous
circulation is not a one-path-only thing: the veins are not like
a tree, where there’s only one path from the trunk to each leaf.
Indeed, this multiplicity of paths is why the problem arises in
the first place: the testicles being drained both by the
spermatic veins and by the local circulation. It’s also why it
can be solved in this manner: the remaining veins enlarge a bit
to accomodate the flow, as well as new veins growing. But it’s
also why they need to be thorough when performing the procedure.
Besides destroying both spermatic veins (a weakness, Gat and
Goren say, of other doctors’ work, where it’s often only done on
the left vein where the swollen veins tend to be more prominent),
they also follow up by injecting more pulses of contrast agent to
make sure that there’s no collateral circulation bypassing the
blocked vein, and that the veins they did treat are really
blocked. (The finger blocking the vein at the bottom keeps the
sclerosing agent where it belongs rather than letting it spread
further into the body. Of course a little of it still spreads,
but the sort of agent they use is harmless at low
concencentrations.)
It’s odd for there to be such an easily-removable design flaw in
the human body; evolution tends to remove them. Since it strikes
at advanced ages, BPH doesn’t make a big impact on a man’s
ability to pass on his genes. But being the leading cause of
male infertility sure does. Their explanation is that evolution
hasn’t had much time to work on the problem; in animals the
spermatic vein is horizontal, and doesn’t have or need one-way
valves. It’s our standing upright that yields the problem; in
evolutionary terms that’s a recent development.
So that’s their theory. There is an obvious question, though,
which mechanically-inclined reviewers might have raised, and
which deserves an answer. It has to do with pressure and height.
As Gat and Goren say, it is a law of physics that pressure within
a fluid increases as one descends deeper into the fluid, in
direct proportion to the depth. (They invoke Bernoulli’s
equation, which also involves the velocity of the fluid, but they
then assume the velocity is negligible, so are using only the
part of the equation that relates pressure to height.)
But they apply this law only to the blood in the spermatic vein.
It is also applicable to the blood in the arteries and to the
blood in other veins. Doing so, though, would ruin their
argument: it would mean that although pressure increases as one
descends down the spermatic vein, it also increases in the other
veins. In their account, blood goes backward through the
spermatic vein, emerging at a pressure of 40 cm H2O or so, then
pours into other veins which, drained by the common iliac vein
which in turn is drained by the inferior vena cava, are at less
than 10 cm H2O. (They use mm Hg as their units, but I’ll be
converting all pressures to cm of water, since it’s a more
directly relevant unit: blood’s density is close to that of
water.)
But gravity acts also on the iliac vein and the vena cava! In
general, if you have a loop of tubing in a gravitational field,
and the fluid inside is not in motion, the pressure of the fluid
is everywhere proportional to altitude, no matter how the tubing
is shaped. No spontaneous circulation arises. Here we have a
loop with the spermatic vein being on one side and the vena cava
and iliac vein on the other, connected on top and bottom, and the
claim is that this is enough to give rise to spontaneous
circulation. It’s not.
Yet there is reverse flow in the spermatic vein when the valves
fail; that’s standard medical knowledge. And, as mentioned, Gat
and Goren confirm the reverse flow by injecting tracer dye into
each and every vein they treat, before sclerosing that vein. The
reverse flow exists; it just has to have a more complicated
explanation than the one they provide.
Another part of their argument is that venous blood infiltrates
the prostate due to the venous pressure exceeding the local
pressure in the arteries that feed the prostate. But gravity
also acts on the blood in arteries. If the local arterial
pressure were equally boosted by height, it could not be
exceeded.
Fortunately for their argument, that’s not the way the human body
works. It sort of theoretically could work that way, if all the
blood vessels were rigid: pressure everywhere would increase with
decreasing altitude in the same way that pressure increases with
depth inside the ocean. Relative pressures would be higher in
arteries, lower in veins, and intermediate in capillaries: the
pressure difference between artery and vein would be the same
throughout the body, but the pressures of both would be highest
in the feet and lowest in the head. (For fish, or babies in the
womb, this works even without blood vessels being rigid, due to
the outside pressure varying with altitude in the same way as the
pressure in their blood vessels does; all the fluids involved
have densities close enough to the density of water that the
difference doesn’t matter.)
Of course in fact blood vessels are not at all rigid. Arteries
are the closest to being rigid; they’re thick-walled and can take
a lot of pressure, though they still have an important amount of
elasticity. Veins, though, are thin-walled: they swell up with
higher pressure and collapse flat at negative pressure.
Capillaries don’t swell, but at higher pressures they leak fluid
into the surrounding tissues, producing swelling there. So the
body maintains capillary pressures in the neighborhood of 20 cm
H2O (somewhat higher at the start of the capillary and somewhat
lower at its exit).
The usual figures for blood pressure (the ones for which “normal”
is about “120 over 80”) are arterial pressures in units of mm Hg,
measured with a blood cuff around the arm (at about the same
altitude as the heart). In that example, the 120 is the maximum
pressure just after the heart beats, and the 80 is the minimum
pressure it reaches between beats. Those numbers translate into
about 160 cm H2O and 110 cm H2O.
In a standing person, the arterial blood pressure is considerably
higher if measured at the ankles, as it would be in the
rigid-vessel model. But then as large arteries divide down into
smaller and smaller arteries, most of that pressure is spent
before it gets to the capillaries. This is not just a matter of
passive friction but also of active control: arteries have
muscular walls and contract to restrict blood flow to the part of
the body that they feed. When more blood flow is needed
somewhere, the local arteries relax to let it through.
Veins operate at low pressure, usually less than 15 cm H2O; the
hard part, and the part that is most relevant here, is explaining
how they get blood upwards against gravity, since their pressure
is nowhere near enough to do so. The one-way valves in the
spermatic vein have already been mentioned, and are present in
many other veins too, but valves themselves don’t do any actual
pumping work (in the physics sense of the word “work”). Yet work
needs to be done to pump blood uphill. In most cases that work
is supplied by muscle movements in nearby muscles, which compress
the veins. In engineering, pumps are often built with two
one-way valves, the volume between those valves being alternately
compressed and expanded; the principle is the same here. But
since it relies on muscle movements which are made for other
purposes, this process is slow and uncertain. So to get the same
throughput the veins are much wider than the arteries.
When the veins’ one-way valves fail near the surfaces of the
legs, it results in high pressures and swollen, unsightly
“varicose veins”. Even with functioning valves, standing
motionless results in high venous pressures in the feet; thus the
advice, on long trips sitting down, to take breaks and to move
the feet and legs around, so as to avoid blood stagnating and
clotting (a “deep vein thrombosis”). For blood circulation
fidgeting is good, even if it’s not “good manners”.
But the vena cava is different: it doesn’t have any valves;
neither does the iliac vein (at least the portion of it under
discussion here). Yet somehow blood which feeds into it at low
pressure manages to climb to the heart, overcoming that 40 mm H2O
pressure difference and more.
I looked in the medical literature for how this actually happens,
and was disappointed not to find a clear, definitive answer.
It’s common to see it stated that breathing has an effect. The
heart is located between the lungs, so shares a common pressure
compartment. The pressure outside the heart is about the same as
the pressure outside the lungs. That pressure decreases to
inhale and increases to exhale. But those pressure excursions
are minor: about 1 cm H2O in either direction. (This rises with
heavy breathing, as in exercise; but the blood circulation has to
work even when the body is calm.)
There is also a disturbing level of confusion in the medical
literature as regards what “zero pressure” is. Good engineering
practice is to say what reference pressure one is measuring
against. Commonly that is the ambient pressure (the pressure of
the air surrounding the body, which is about 1000 cm H2O at sea
level and lower at higher altitudes). To inhale, one obviously
has to drop the pressure in the lungs below ambient pressure. A
measurement relative to ambient pressure is called “gauge
pressure” by engineers, because it’s what the usual sorts of
pressure gauges tell you. The old-fashioned mercury manometers
for measuring blood pressure measured gauge pressure, since they
were open to the air on their other end. Modern mercury-free
manometers are no doubt built to give the same numbers (so as to
be compatible, and also because it’s easy to build a gauge that
way.) So the usual arterial blood pressure numbers are all
referenced against ambient pressure.
That is so even if medical literature tries to confuse the
matter, as happens. Take, for instance, this passage in Guyton
and Hall’s Textbook of Medical Physiology, 14th edition (a
standard text in medical schools):
Pressure Reference Level for Measuring Venous and
Other Circulatory Pressures Although we have spoken of
right atrial pressure as being 0 mm Hg and arterial pressure
as being 100 mm Hg, we have not stated the gravitational
level in the circulatory system to which this pressure is
referred. There is one point in the circulatory system at which
gravitational pressure factors caused by changes in body
position of a healthy person usually do not affect the pressure
measurement by more than 1 to 2 mm Hg. This is at or near
the level of the tricuspid valve, as shown by the crossed
axes in Figure 15-12. Therefore, all circulatory pressure
measurements discussed in this text are referred to this level,
which is called the reference level for pressure measurement…When a person is lying on his or her back, the tricuspid
valve is located at almost exactly 60% of the chest thickness
in front of the back. This is the zero pressure reference level
for a person lying down.
Now, what is meant by that? It purports to define a pressure
reference level, and one that is not just equal to ambient
pressure. But does it mean that every single blood pressure
measurement in the entire book was done by snaking a catheter
into the heart until it reaches the tricuspid valve, and using
that as reference? Of course not! That would be ridiculously
dangerous and expensive. Also, that valve is in the middle of
the heart: it’s the valve between the right atrium and the right
ventricle. The pressure there varies strongly as the heart
beats, making it unsuitable as an experimental reference
pressure.
So the passage cannot really mean that it is defining a reference
pressure. Instead it is defining a pressure reference
altitude. I take it to be just an overly fancy way of
standardizing the practice of putting the blood pressure cuff
around the top of the arm with the arm draping normally down the
side of the body, which puts the cuff at about heart height.
(Even on someone lying down, that position of the cuff is still
at about heart height.)
Unfortunately it also betrays a lack of real precision, which is
probably unimportant as regards the usual use (arterial
pressures), since those numbers are large, but is important when
dealing with the much lower pressures in veins and the issue of
how venous blood is pushed back from the lower body to the heart.
This lack of a definition also extends to the Gat and Goren
papers: they give pressure numbers, but never say relative to
what. Ambient pressure generally seems like the best reference
pressure to use; I will be hoping that papers I cite for
pressures used it, but also, since none of them say they do, not
placing much trust in any absolute numbers.
Enough complaining; time to return to the question of how blood
makes it back to the heart. As mentioned, inhaling a breath
reduces the pressure in the heart and lungs; but that reduced
pressure ends at the diaphragm, the sheet of muscle and tendon
that separates the thorax (heart and lungs) from the abdomen
(guts). The diaphragm is arched upwards; to inhale we contract
it, flattening it and lowering the pressure in the heart and
lungs while increasing the pressure in the abdomen. Those two
pressure changes add together to force blood in the vena cava up
past the diaphragm toward the heart. (This is for normal
breathing; in general the pressure in the abdomen can vary quite
considerably depending on what the abdominal muscles are doing,
as measured for instance in this paper. Above the
diaphragm, it also depends on what the rib muscles are doing,
they being another force that powers breathing. But in normal
breathing the diaphragm is the main actor.)
So breathing helps as regards the general problem of returning
blood to the heart. Still, the heart does need blood feeding it
when breathing out as well as when breathing in. We don’t
synchronize our breaths with our heartbeats, nor could we, since
breaths are less frequent. (The frequency of the heartbeat does
change a bit in breathing out compared to breathing in, though.)
It helps that the lungs are elastic, and would collapse if there
weren’t more pressure inside them than outside. This means the
normal pressure in the thorax is negative, helping to pull blood
in. This “transpulmonary pressure” (the difference between the
air pressure in the lung and the fluid pressure outside it) is
about 6 cm H2O.
The heart, as it relaxes from its contraction, presumably also
can exert a bit of suction on the incoming flow; it’s obviously
not rigid enough to exert much suction, but every little bit
helps.
That the system operates right on the edge, getting blood back to
the heart with almost no pressure to spare, can be seen by the
reader if he examines the veins on the back of his hand: if held
at lap level those veins stand out, full of blood, while if
raised to eye level they flatten. By moving the hand up or down
slowly, giving the veins time to empty or fill, the level where
the transition from full to flat occurs can be determined; it’s
about at the same level as the heart. (This demonstration
probably won’t work for everyone; blood vessels are less
prominent in the young, and also can be hidden by fat.)
Now to return to the main question here, which is backflow in the
spermatic veins. Those veins are entirely below the diaphragm.
They are in the same pressure compartment as the vena cava is at
that altitude (the retroperitoneal space), so the two competing
flow paths are under about the same pressures.
I’m afraid that the answer is that Gat and Goren are simply wrong
about the pressure at the bottom of the vena cava when standing:
it must be at considerably higher pressure than the sub-10-cm H2O
that they say it is – or at least it is when the patient is
standing up (or sitting normally). It’s an understandable error,
since the medical literature usually refers to the lower numbers:
in circumstances where it matters, patients are commonly
horizontal.
I found a paper from 1966 which measured intravenous
pressures both with the patient horizontal and with the patient
standing: the horizontal numbers are in accordance with Gat and
Goren’s, but the standing numbers are considerably higher. Using
their numbers for the common iliac vein (since they don’t say
where in the vena cava they measured pressures, and since the
common iliac vein is where the veins from the prostate enter, so
is the most relevant number anyway), the average of their three
measurements has the pressure with patient horizontal as 8 cm H2O
(plus an additional 9 cm H2O when breathing in), and the pressure
with patient erect as 30 cm H2O (plus an additional 15 cm H2O
when breathing in). If we accept these numbers, the pressure in
the common iliac vein is close to the pressure in the bottom of
the spermatic veins.
Again, though, it’s generally agreed that the backflow exists, at
least through the spermatic vein. (Gat and Goren’s contribution
is extending it to the prostate.) The large pressure difference
they say is driving the backflow is calculated based on improper
assumptions, but there must be some pressure difference driving
the backflow.
Now, one difference between the two veins competing for flow here
is that the vena cava is much larger and thus has much less
frictional resistance to flow. In general, blood flow in the
body is dominated by frictional effects (fluid friction, that is,
aka viscosity): Gat and Goren cite Poiseuille’s equation for
fluid flow, which states that flow is proportional to the fourth
power of the diameter of the pipe. In comparison, if friction
were zero, the flow would only be proportional to the square of
the diameter of the pipe (that is, proportional to its
cross-sectional area). But Poiseuille’s equation, which has it
as the fourth power, is the generally-accepted formula for blood
flows. It’s just an approximation, not a fundamental law, but
the fact that it’s the accepted approximation shows how dominant
friction is in blood flow.
The ratio in diameters between the spermatic vein and the vena
cava is about a factor of 7. So if equal lengths of the two
veins are subjected to the same pressures, Poiseuille’s equation
says that the vena cava has 2401 times the flow and 49 times the
average flow velocity. But that just predicts much less forward
flow in the spermatic vein; it does not predict that it
backflows.
Yet it does. The hydrostatics don’t predict it, though; we need
to look into its hydrodynamics. The two main sources of dynamic
behavior (pulsations or fluctuations rather than constant flows
and pressures) have already been mentioned: one is the right
atrium, alternately accepting inflow then contracting in the
heartbeat, its input valve slamming shut to prevent backflow.
The other source is the breathing.
Since intestines have basically nothing holding them in place (as
can be attested to by any hunter who has gutted an animal,
slitting open its belly and seeing the guts spill out), it’s not
just the blood in the veins but the whole abdominal compartment
that is reasonably well modeled as having a pressure gradient
determined by altitude: about one cm H2O pressure for every cm of
altitude (since most things in the body have a density close to
that of water). Of course pressures inside a pressurized cavity
(such as the bladder, a vein, or an intestine) can be higher, but
even within those cavities there is the same pressure gradient
with altitude (unless filled with air or some other gas, as
intestines sometimes are).
Though I earlier alluded to the retroperitoneal space as a
pressure compartment, the peritoneum is a thin membrane which, in
surgery, tears easily; there’s not much chance of it
holding significant pressure for long. What mainly holds in
abdominal pressure are layers of muscle and connective tissue.
In the bottom of the abdomen this is the pelvic floor muscles.
In men, those are just below the prostate, so the prostate too is
in the same pressure compartment.
What isn’t in that pressure compartment are the testicles; they
are hanging out in the breeze, surrounded by ambient pressure.
The level of influence that the heart’s beating has on the
pressure in the vena cava can be judged by the velocity of flow
in it. According to measurements done in this paper, the
velocity in the inferior vena cava peaks at 45 cm/second (in
people breathing normally while lying down, and measured at the
level of the T11-T12 intervertebral disc). Per Bernoulli’s
equation, that energy density is the same as is produced by a
pressure rise of 2 cm against Earth’s gravity. (This is also the
height an object rises to if it is thrown upwards with that
speed.)
2 cm H2O is not much pressure rise, particularly compared to the
above-cited 15 cm H2O pressure increase in the abdomen for
breathing in. Also, the pressure pulse from the heartbeat is
entirely inside the veins (and diminishes with distance from the
heart), whereas the 15 cm H2O pressure increase is applied to the
whole compartment uniformly. (But neither of those numbers is
particularly certain, and both must vary considerably depending
on circumstances.)
As mentioned above, capillaries and veins can only hold limited
blood pressure. But what matters is the difference between
external and internal pressure. If capillaries exceed maybe 40
cm H2O, they leak fluid, producing edema; but inside a pressure
compartment at 30 cm H2O that internal pressure limit increases
to 70 cm H2O. Numbers like those occur near the bottom of the
abdominal cavity when standing: pressures higher all-around
without producing distress. But if the testicles, which are
outside that pressure compartment, experience the same internal
pressure, that will produce distress in capillaries and veins.
While intact, the spermatic vein is protective: its one-way
valves mean that blood is drawn upwards when the abdominal
pressure is at its minimum and can’t return when it’s at its
maximum. Still, to get into the abdomen at all, the blood in the
spermatic vein has to be at least at the pressure of the abdomen
(at the point of entry, which here is near the bottom of the
abdomen). The abdominal pressure varies; if the pressure in the
spermatic vein is between the minimum and the maximum abdominal
pressures, then the part of the vein which is in the abdomen will
be collapsed part of the time, which with intact valves makes for
effective pumping. If the pressure in the spermatic vein is
above the maximum abdominal pressure, it doesn’t need pumping to
flow, but still functions as a pump, via the vein expanding and
contracting.
So when the one-way valves fail, the pressure at the bottom of
the spermatic vein increases. Still, the difference in pressure
between the failed and working states is not the full 40 cm H2O
that Gat and Goren deduce from the height of the spermatic vein,
but rather cannot be more than the 15 cm H2O pressure difference
between breathing in and breathing out.
Now, that last number is just a measurement from one paper,
likely from one single Irishman; and if he had been breathing
harder the number would have been higher. So it should not be
taken as gospel; but it wasn’t easy even finding that number.
Not that I pretend to thoroughness; I could well have missed a
better source, and readers who know of one are invited to provide
it. But I’ve spent enough time looking that at this point it
seems like it might be easier to measure intra-abdominal pressure
changes myself, sticking a pressure probe up my butt – that’s
literally how this paper did it (but on horizontal patients
rather than vertical ones, so their numbers are not quite on
point here.) That’s not quite the same thing that the Irish
paper measured, which was intravenous pressure; sticking a
catheter into my veins is way beyond my skill set. But for
measuring pressure increase it’s close enough, since the
pressure increase from breathing is applied to the whole
compartment.
In any case, that’s why there’s higher pressure at the bottom of
the spermatic vein when the valves fail, but it doesn’t answer
the question of why there is backflow. The situation, again, is
of two vertical veins, connected at top and bottom, in the same
pressure compartment, with one vein being much larger than the
other. Static, constant flow would just be divided between the
two; both would flow upwards. But consider rhythmically varying
flow, such as is produced by the heartbeat or by breathing.
First there is an acceleration phase, which acts on both veins.
In the vena cava, with much less friction, the blood accelerates
upwards, whereas in the spermatic vein, with more friction, there
is much less acceleration; the extra upwards force is mostly
spent against friction. Then there’s a deceleration phase. The
blood in the vena cava has inertia; it’s trying to go upwards but
has to stop, so the pressure at its top increases. Some of that
higher-pressure blood then is pushed down the spermatic vein.
And the flow up the vena cava has such a dominant share of the
overall flow that even a slight influence like this can yield net
backflow in the spermatic vein.
Now, the mechanism I just described does not seem like a
particularly strong mechanism; but as mentioned, in practice it’s
well established that there is backflow; this is just one way
that theory can agree with that observation. There may be other
mechanisms that do better, perhaps involving the portion of the
flow which is outside the abdominal pressure compartment, and/or
the relatively narrow veins communicating between the two paths
at the their bottom – though that gets complicated enough that
it’s not easy to think through; a computer model might be in
order.
More questionable, although still quite plausible, is the idea
that the backflow reaches the prostate. The Gat and Goren theory
is that it is pushed by about 40 cm H2O pressure, overwhelming
even the prostate’s arterial supply. But that’s pure theory;
they don’t report actually measuring any pressures. (If they
were researchers I would ding them harshly for not doing the
measurements, but they’re clinicians; they’re paid to cure
patients, not to produce knowledge.)
Gat and Goren didn’t do a great job of proving experimentally
that backflow reaches the prostate, either. They measured the
high free testosterone levels described above, but that
measurement was at the bottom of the spermatic vein; they didn’t
have their catheter make another turn and go into the deferential
vein and then past its junction with the vesicular vein and into
the vein coming from the prostate, so as to measure high free
testosterone levels in a place where they definitely don’t
belong. (This is readily excusable: those other veins are much
smaller, so the catheter might not fit or might not be able to
make the sharp turn into the smaller vein. Again, researchers
should be dinged for omitting such tests – there’s got to be
some way to measure pressures there – but clinicians get a
pass.)
Perhaps the strongest piece of evidence they offer for backflow
to the prostate is an X-ray showing contrast agent which was
injected into the spermatic vein. They state that:
On retrograde venography of the PP, after a
delay of about 10 s, a contrast material ‘blush’, of the
prostate gland capsular region, was observed (Fig. 3).
Both are clearly seen in the image.
Here’s their Figure 3 (again, reproduced from their most recent
paper under the terms of a Creative Commons license):
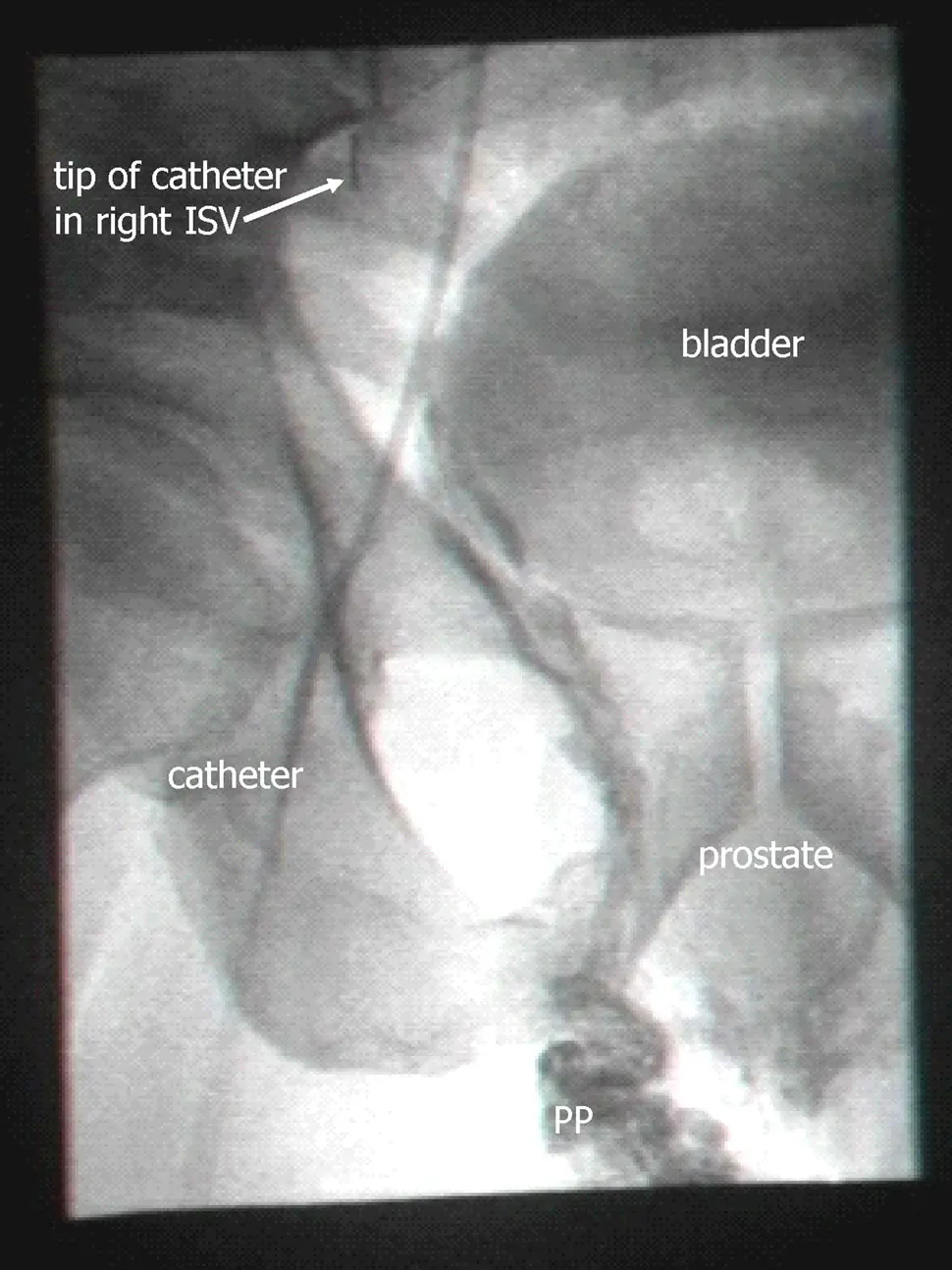
Looking at it, I can see the oval they label “prostate”, but it’s
pretty faint. There are various other structures (such as the
bladder) which are also visible with similar intensity. Backflow
down the spermatic vein is clearly visible (all that darkness
going down to the region marked PP, for “pampiniform plexus”, the
network of blood vessels above the testicle), but backflow down
the spermatic vein is not the new part of their theory. There is
some darkness under the the prostate, which looks like
backflowing dye, but is not clearly connected to the dye in the
PP region. Even if it is dye, it’s not evident whether it gets
into the prostate itself. Perhaps specialists who are used to
viewing fluoroscopy images will pronounce this one definitive,
but for me it’d take a movie showing contrast emerging from the
tip of the catheter and then making its way to the prostate; in
this day and age, movies should be easy to provide. Of course in
their clinical practice they are seeing such a movie with their
own eyes for every patient, so it requires a modest level of
distrust to doubt them on this. But it’s not entirely unknown
for doctors to succumb to wishful thinking, and “blush” is not a
strong word. I’m not inclined to distrust them on this, but the
image they show is less than a complete proof.
I’d also be curious, with such a movie, to look for variations in
the flow. There would no doubt be motion visible from breathing,
and the contrast agent would show how that correlated with blood
flow in the veins.
Breathing would help spread the testosterone-loaded outflow,
since the prostate is inside the abdominal pressure compartment
while the testicles are outside. Breathing would thus push the
flow alternately in one direction then the other, increasing the
chance of subjecting the prostate to outflow from the testicles.
The movie would show this. Even if it resulted in only a weak
and diluted flow which didn’t deliver the theorized hundred-fold
increase in free testosterone, a ten-fold increase is still quite
a lot and even a doubling would be important.
A really thorough experimental workup would measure all the
relevant flows and pressures, perhaps by using an intravenous
catheter loaded with multiple pressure and flow sensors each
delivering many measurements per second. (Whether such a
catheter can be bought today and whether it can be readily
inserted into the veins in question are not matters I have looked
into, but with modern technology there’s got to be a way of
measuring such pressures.) Testosterone levels would also be
measured at various points.
Recently a paper by Alyamani et al came out which did do
such measurements of testosterone. The patients were 266 men
undergoing prostatectomies for prostate cancer, none of whom had
any prior hormonal therapy. During the course of those
operations the researchers extracted blood from the prostatic
dorsal vein and tested it for testosterone. They also tested the
removed prostates for testosterone. They indeed found levels of
testosterone in the dorsal vein and in the prostate which were
many times higher than the levels measured in normal peripheral
blood from the same patient. Related substances in the samples
were consistent with the blood having come straight from the
testicles. They dubbed this phenomenon “sneaky T”, and describe
their results as supportive of the Gat/Goren theory.
If I were just being booster-ish, I could leave it at that (“yay,
support”); but I’m taking a hard numerical look. And from a
numerical point of view, at first glance that support seems weak.
Only 20% of the men had dorsal vein / peripheral vein ratios of
testosterone which were two or greater. About half of them had
ratios below one. This is not what you’d expect if this were the
main driving force of prostate cancer: you’d expect seriously
elevated ratios in pretty much all the men, not just in 20% or
50% of them.
The association they found between dorsal vein testosterone and
prostate tissue testosterone is also weak. By biology standards
it’s totally there (P = 0.004), but there were still a lot of men
with high levels in one of those places and low levels in the
other. This is not what you want for the definitive mechanical
cause of something.
But the title of a paper they cite caught my eye:
“Testosterone and estradiol are co-secreted episodically by the
human testis.” Looking at it, I find it could explain the above
weaknesses: the testicles don’t put out testosterone all the
time, but rather do so only in pulses, at the rate of about one
pulse per hour. Testosterone stays in the bloodstream long
enough that testosterone measurements in circulating blood don’t
vary much, but the output of the testicles varies quite a lot.
The paper’s measurements (once per fifteen minutes) weren’t
frequent enough to really pin down the length of each pulse, and
accordingly they give no figures for that length; but eyeballing
their graphs, maybe elevated testosterone is present about a
quarter of the time, though it’s hard to tell.
So when the Alyamani paper found doubled levels in 20% of men,
that could mean that it was actually happening in all of them,
just only about 20% of the time in each. Or it might be
happening in half of them, about 40% of the time in each. Also,
the stress of surgery might have affected the timing of the
testosterone pulses.
The pulsing also could explain prostate tissue concentrations
often being different from dorsal vein concentrations:
testosterone takes time to sink in, so if they measured on the
leading edge of a pulse the tissue concentrations would be lower
than the vein concentrations, whereas on the trailing edge they’d
be higher. Or the measurements might have been done at different
times during the course of the surgery; the dorsal vein blood
might have been extracted, say, ten minutes prior to the
prostate’s extraction.
So the Alyamani paper’s measurements are consistent with backflow
of high-testosterone blood being a factor not just in 20% or 50%
of cases but in all of them. Still, the backflow, when present,
is doubtless stronger in some men and weaker in others. And
indeed being in that top 20% did have some predictive value:
prostate cancer recurrences were more common in the 20% group.
(Since the prostates themselves were all removed, this presumably
represents metastases. It would be interesting to know whether
the increased metastases were in the same local area bathed by
high testosterone or whether they were distant; but the paper
does not report this.) They also did not measure free
testosterone, just total testosterone, so that’s another unknown.
Besides the BPH paper, Gat and Goren even tried their method on
outright prostate cancer (early stage, Gleason 3+3) and
report disappearance of cancer (as measured via biopsy) in 5 out
of 6 patients, along with lowering of PSA levels.
One thing to note, though, is that their fix of destroying the
spermatic vein and any collaterals can’t make the system as good
as it was originally. Destroying a backflowing vein always
provides improvement in pressures (since otherwise the vein
wouldn’t be backflowing), but it can’t improve pressures as much
as forward flow would. The ideal would be valve repair or
replacement; that is done routinely on heart valves, but not on
vein valves. (This also explains why evolution hasn’t simply
removed the spermatic vein in us: when it’s there and working, it
helps.)
Operations for varicocele are done widely, to try to fix male
fertility, but aren’t always of this sort: it’s common to see the
swollen veins in the scrotum as the problem and simply remove
many of them surgically. This does not squarely address the
problem of backflow, and indeed there’s a bit of debate in the
medical literature over whether it produces statistically
significant fertility improvements. (On the whole it seems to,
but there are enough failures to produce debate.)
Even among doctors using the embolization approach (that being
the term for inserting a catheter and using it to apply a
sclerosing agent), many do it only on the left side, that usually
being the one with the visible varicocele. (There is an
asymmetry among the spermatic veins, with the left vein being a
bit longer and thus more problematic.) Besides doing both sides
(or at least checking both sides for backflow with contrast
agent), sclerosing each from top to bottom, the full Gat/Goren
procedure is also to check for collateral veins and sclerose
those too, and then follow up with more contrast agent to ensure
that it was done properly.
So there’s a chance that a varicocele surgeon will do this
properly, but one can’t just go to a random varicocele surgeon
and assume he’ll do it properly. Even someone who does believe
the Gat/Goren theory can screw it up in practice, especially if
you’ve just got done persuading him and this is his first try.
Screening for this disorder is simple: use a thermal camera and
compare testicular temperature sitting up (or standing) versus
lying down, in each case waiting five minutes or so for
temperatures to equilibrate, and taping the penis up so that it
does not affect the measurement. According to Gat and
Goren, this is almost as good a test as measuring backflow
on fluoroscopy, and a far more sensitive test than trying to look
for the enlarged veins of varicocele. (Rather than a thermal
camera, they use a liquid crystal strip which is designed and
branded for this exact job; but given how expensive medical items
are, these days thermal cameras are likely cheaper and are
certainly more widely available. Their calibration is probably
not as good, though.)
In the above-referenced blog post, Scott Alexander goes on to
write that neglect of this sort of new idea isn’t some sort of
conspiracy; it’s just the default. For starters, most new ideas
everywhere are wrong and deserve to be neglected. And here it’s
not like drugs where the patent system incentivizes people to
develop new ones and gets them enough money to test them
thoroughly and then pay for billions of dollars in advertising.
It also is not one of the “hot” fields in science where
scientists are racing to study the possibilities. It’s not
genomics or the microbiome or any other “-ome”; it’s just
plumbing.
He might have added that most of society’s attention on medicine
is focused on squabbling about who pays for it, with precious
little attention given to possibilities of finding new ways to
actually cure disease. Yet those can represent the most dramatic
cost savings; here, a thousand-dollar procedure might mean not
spending hundreds of thousands of dollars on cancer. The
thinking by the powers-that-be should not always be “oh, no, not
another thing that we’ll have to spend even more money on”,
even when that is true in the short run.
Malpractice law also stacks the deck against innovation: it
doesn’t blame doctors for doing what doctors generally believe in
doing (the “standard of care”), even if the outcome is bad, but
it does blame them in the event of a bad outcome from something
new and innovative. (Which in a way it should, since most new
ideas are wrong; but it’s never really fair to put only the
failures in front of a scientifically ignorant jury and invite
them to shell out millions in damages.)
Insurance doesn’t pay for “experimental” treatments, either.
Advertising by doctors is also strongly frowned-on, particularly
advertising that goes against conventional wisdom; a doctor who
blanketed the local airwaves with claims of a prostate cancer
cure (or even a preventative) would very likely get his license
yanked by the state medical board. (Not that broadcast
advertising really would be the way here: too costly for an
individual doctor. The modern way would be targeted Facebook
ads, relying on Facebook’s intimate knowledge of personal medical
problems. The medical board might not even find out about it,
though if they did they likely would be extra wrathful.)
The back-to-nature crowd is also not interested, at least not in
the financial sense of “interested”, because there’s nothing here
they can sell as a “dietary supplement” and they aren’t permitted
to perform medical procedures. Perhaps they could advocate going
back to walking on all fours? Nah, too impractical. Going back
to the oceans and living like fish, with a supportive pressure
gradient all around us? Nah, even more impractical (though there
already is a sect of “aquatic ape” believers out there).
None of this amounts to conspiracy – it’s all in the open – but
it adds up to quite a set of obstacles. (It’s not like anyone’s
paying me to write this, or like I have much prospect of making
money from it; it’s just the strong curiosity of someone whose
grandfathers both died from prostate cancer.)
Being surgery, this procedure doesn’t need to go through FDA
approval, so there’s no decade-long process to form an obstacle
to people getting it. Finding a doctor who will do a good job of
it, though, is not simple; a short glance at patient forums
reveals considerable frustration. I can’t tell if the Gat/Goren
clinic in Israel is still open for business: their website
www.pirion.co.il isn’t responding, and given those doctors’ ages
it wouldn’t be surprising if it has closed. The German clinic
which did the 2014 reproducing study might still be doing
the procedure. At any rate, though, a single clinic can’t handle
worldwide demand for such a common disorder – not even just
elite demand.
The procedure also doesn’t last forever, according to Gat and
Goren’s latest paper. They write that new venous bypasses
grow to replace the destroyed spermatic veins: at first these are
tiny, but then grow to where the problem recurs. Part of their
reasoning, though, is faulty: they write that at first the size
of each growing vein is small enough that capillary action can
draw fluid up. Now, it’s quite true that capillary action (the
physical/chemical attraction between the fluid and the walls of
the capillary tube) can draw fluid up through great heights;
trees, for instance, rely on it to draw water into the treetops.
But it can only draw fluid up into empty space; it has no
lifting effect when the tube starts out completely filled, as is
the case here. (In trees it’s the evaporation at the top that
powers it.) A simpler explanation is just that those tiny veins
are too small to cause the problem to recur. In any case, the
paper makes no comment as to whether the problem can be solved
the same way a second time; obviously in principle it can, but
finding all the new bypasses and sclerosing them might be
difficult in practice.
As for mass adoption, I’m well aware that this essay is not the
stuff of a mass patient movement. I’ve tried to make it
accessible, but the needs of actually being accurate and making a
thorough technical argument mean that not more than one in a
thousand patients would read to the end of it. (If you think
otherwise, please consider that your social circles are not
typical.) A video with animations would improve that proportion
considerably, maybe to one in a hundred, and I’ve considered it,
though I’m not handy with animation tools. But it’ll take
serious interest from scientists and doctors to really put an end
to all this prostate trouble.